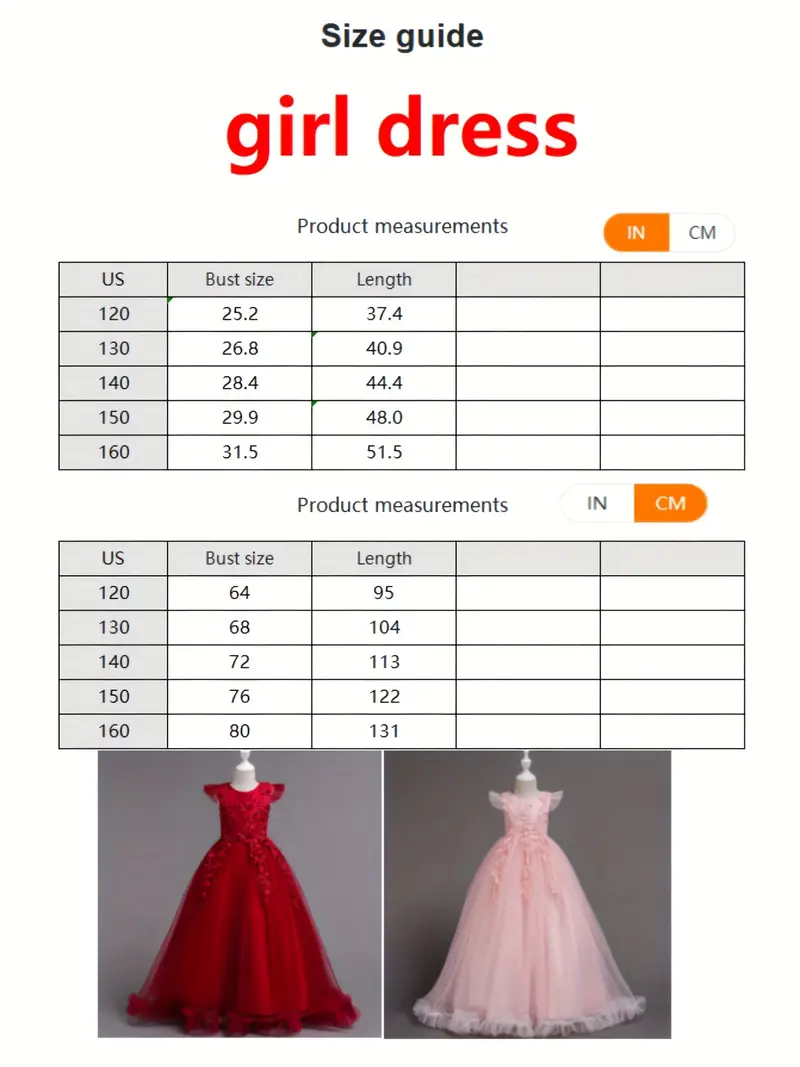
Świąteczna Sukienka Księżniczki Dla Dziewczynek Śnieżna Graficzna Siatkowa Tiulowa Sukienka Maxi Bez Rękawów Wesele Formalna Suknia Do Tańca - Temu Poland

Kidsbutik.pl - Ubranka dla dzieci i Zabawki > Sukienka świąteczna czerwona krata Zestaw Rodzinny - tu córka
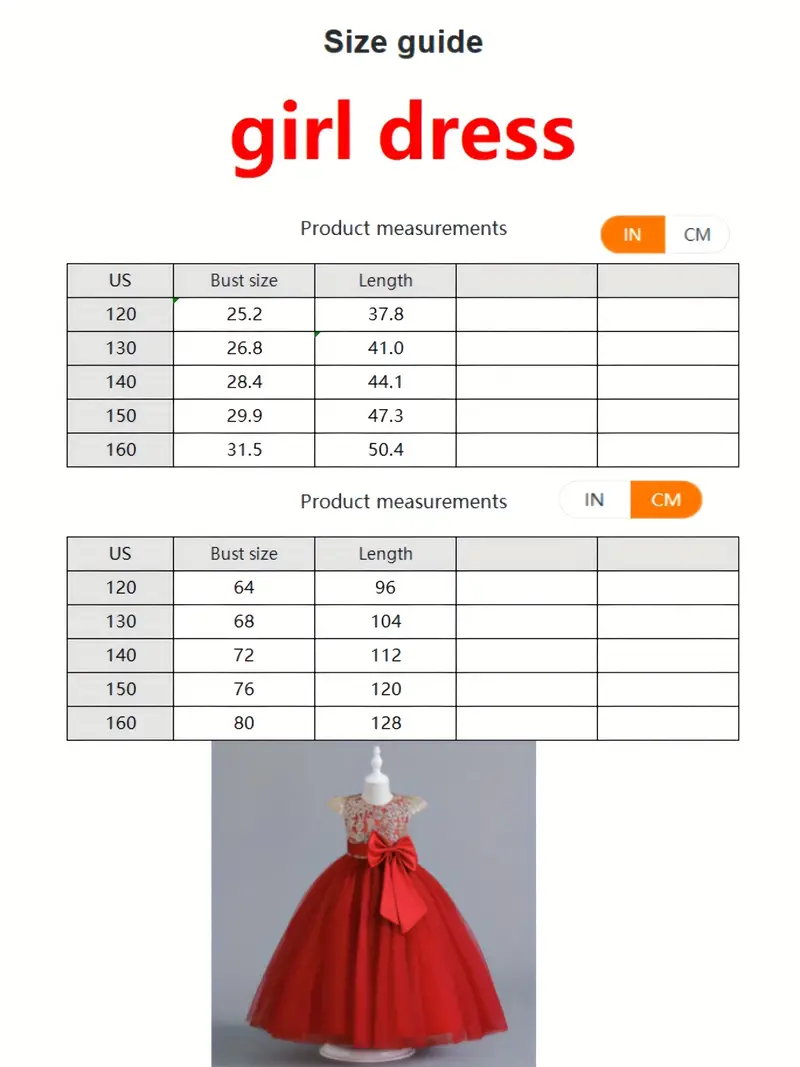
Świąteczna Sukienka Księżniczki Dla Dziewczynek Śnieżna Graficzna Siatkowa Tiulowa Sukienka Maxi Bez Rękawów Wesele Formalna Suknia Do Tańca - Temu Poland

Sukienka świąteczna dla dziewczynki czerwona kratka biała tutu tiul ŚWIĘTA 104 110 - Sklep Sell4KIDS

Sukienka świąteczna dla dziewczynki MIKOŁAJKA z opaską 80 86 92 98 104 110 PREZENT - Sklep Sell4KIDS





















